The medical industry has always been one of the most significant and bustling elements of the world economy. This is due to their effects in reducing costs, increasing access, and enhancing treatment options and illness prevention. The healthcare market mainly consists of hospital care, technology, and notably medicine. In 2022, the market was valued at $1.48 trillion and is projected to have a revenue of $60.72 billion in 2023.
Despite the massive revenue generated by each year, its development expenses are immense. A compound must pass a long period of animal testing before it is legally available to humans; however, the difference in animal and human physiology results in inefficiencies and harm. According to Harvard University, the United States spent almost $125 billion on drug development in 2019, which only resulted in 48 medicine registered. Additionally, it takes up to ten years to develop one, and its failure rate is up to 90%.
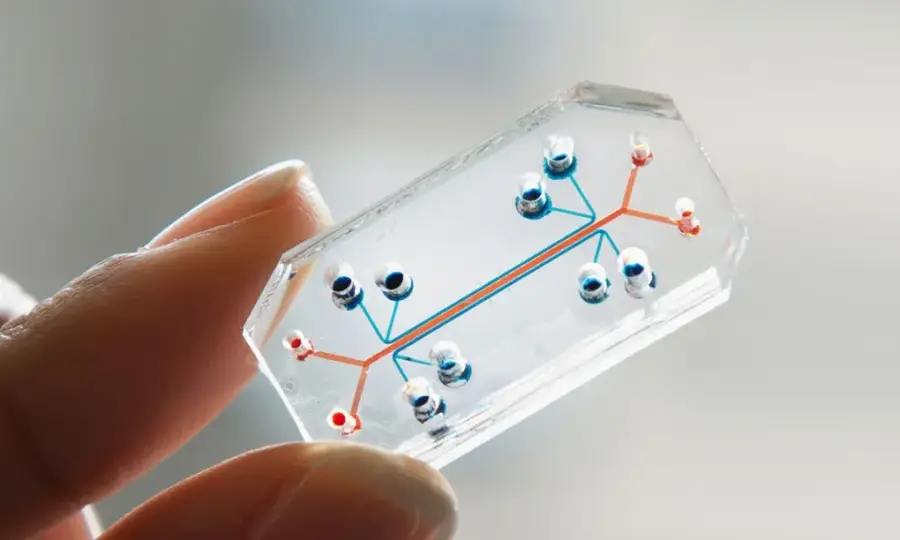
In 2007, Wyss Institute invented organ-on-a-chip (OoC), a device containing cell culture imitating human organs. This invention allows the control of biochemical and cellular responses, which are not available in animal testing. Combined with new technology (e.g. AI), organ-on-a-chip can revolutionize pharmaceutical development for the better.
Table of Contents
The Painful Process of Drug Development
From a medical standpoint, traditional drug development is considered to be extremely complex and costly primarily due to its processes and low success rate. Despite the technological advancements, pharmaceutical advancements remained constant.
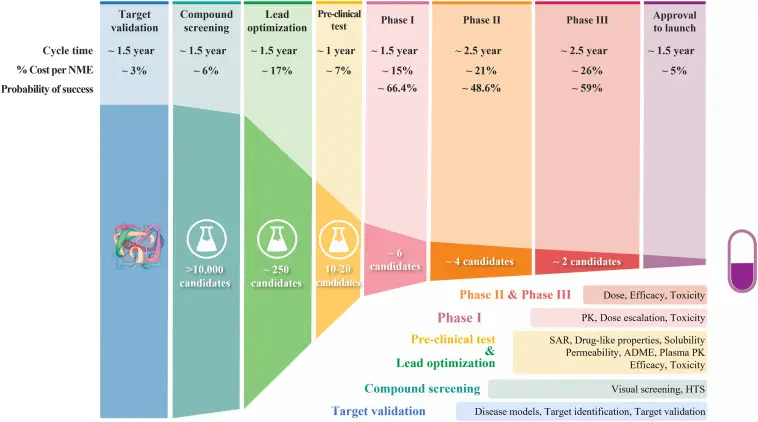
To initiate drug development, researchers must recognize a disease and its biological functions to pinpoint suitable compounds. The process can take up years and millions of dollars to develop the medication and get the approval of medical agencies; subsequently, the compounds are tested on organisms to determine their efficiency and safety for clinical trials.
After preclinical testing, the drugs are tested on human participants to confirm their safeness, effects, and dosage on patients. Trials result in years of testing and costly expenses in recruitment, testing, and monitoring hundreds to thousands of patients. Once the examination is completed, agencies must submit their data to the FDA for assessment. Each submission cost $3.1 million and is often lengthy and complex. If the drug is authorized, it is ready for commercialization.
Finally, the compound is made available for the market, and manufacturers must monitor the drug’s safety and efficiency on their customers. The production cost of a medication is high due to its complexity and the techniques required. Moreover, agencies must invest in patent protection, legal fees, and litigation costs to prevent legal complications. According to Duxin Sun, approximately 10% of compounds made it to the last stage.
How Does a Lung Fit in a Chip?

The concept of OoC originated in the 20th century, but its development began in the 2000s. Microfluidics is made available by technological advancements in the 21st century, allowing the creation of miniature cellular systems. 2001 marks a giant step in microfluidics when George M. Whitesides and his colleagues demonstrated its utility in cellular and tissue functions, such as fluid exchanges between cells.
We took a game-changing advance in microengineering made in our academic lab, and in just a handful of years, turned it into a technology that is now poised to have a major impact on society.
Donald Ingber
Shuichi Takayama, a Japanese chemist, joined Jim Grotberg’s research in biomedical microfluidic devices. Takayama partnered with Dongeun “Dan” Huh, a student in Grotberg’s department. They managed to produce the first OoC in 2005 with “beautiful” success, according to Huh. After a year, the research team was invited to Donald Ingber’s lab due to his interest in microfluidics.
During Huh and Ingberg’s demonstrations in 2007, the OoC successfully replicated the impacts of edema and gained appraisal from senior scientists. NASA and various agencies are interested in its usage according to Albert Folch. In 2009, Ingber launched the Wyss Institute to research biomimetic technologies, including OoC.
In 2010, the Wyss Institute publicly introduced the lung-on-a-chip. Wyss Institute stated that the main purpose of OoC is to improve, accelerate, and lower the cost of drug development and understand the human physique. Additionally, the Wyss Institute added that the transparency of the chips allows scientists to see the effects of medications, toxins, or diseases.
In October 2022, the agency produced 15 models of human organs on OoC, such as intestines, kidneys, or skin.
They can all be connected to stimulate human bodily functions.
Cutting the Middle Rat
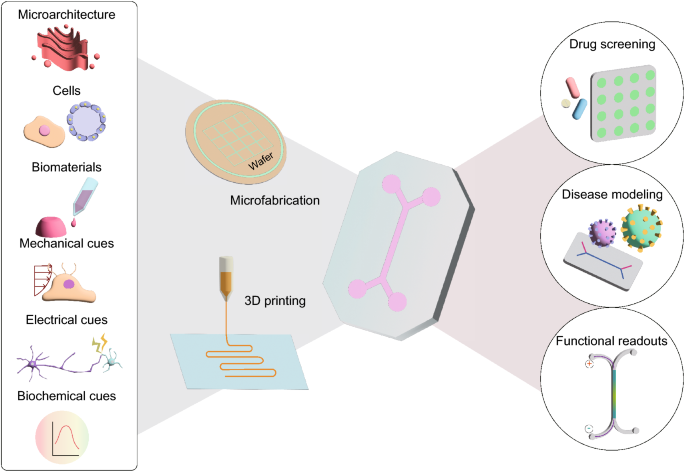
The advent of organ-on-a-chip can revolutionize drug development and profit medical companies. Due to their human-like structures and variants, the advantages of OoC outweigh traditional animal testing. Thus, many companies began to invest and lobby for OoC technology.
Experimenting on nonhuman subjects can result in miscalculations in the efficiency and safety of a drug in humans. On the other hand, OoC provides realistic and accurate results in drug testing. Utilizing OoC in preclinical trials can reduce the amount of time and funds spent on test subjects for a drug to commercialize.
Animal models can inhibit dissimilar effects of a condition which can hinder research and development of medications. Since the OoC are designed after organs, they can reflect symptoms clearly without external involvement. The invention can accelerate experimentation on new compounds and therapies. Likewise, the long-term effects of a medication are more detectable, increasing drug safety and lowering liability costs.
The production cost of an organ-on-a-chip is inexpensive, reducing development expenses and increasing efficiency. Agencies can conduct massive tests on OoC without financial liability and inaccuracies. Drug development will become more streamlined, resulting in more compounds being produced, thus more revenue for the companies. In addition, OoC will cut the ethical issues of drug testing entirely, namely animal testing.
Overall, the pharmaceutical industry will be influenced by the ever-growing organ-on-chip technology which can reduce the development costs of compounds, enhance drug development, and improve medication safety and efficiency.

